DNA mismatch repair (MMR)
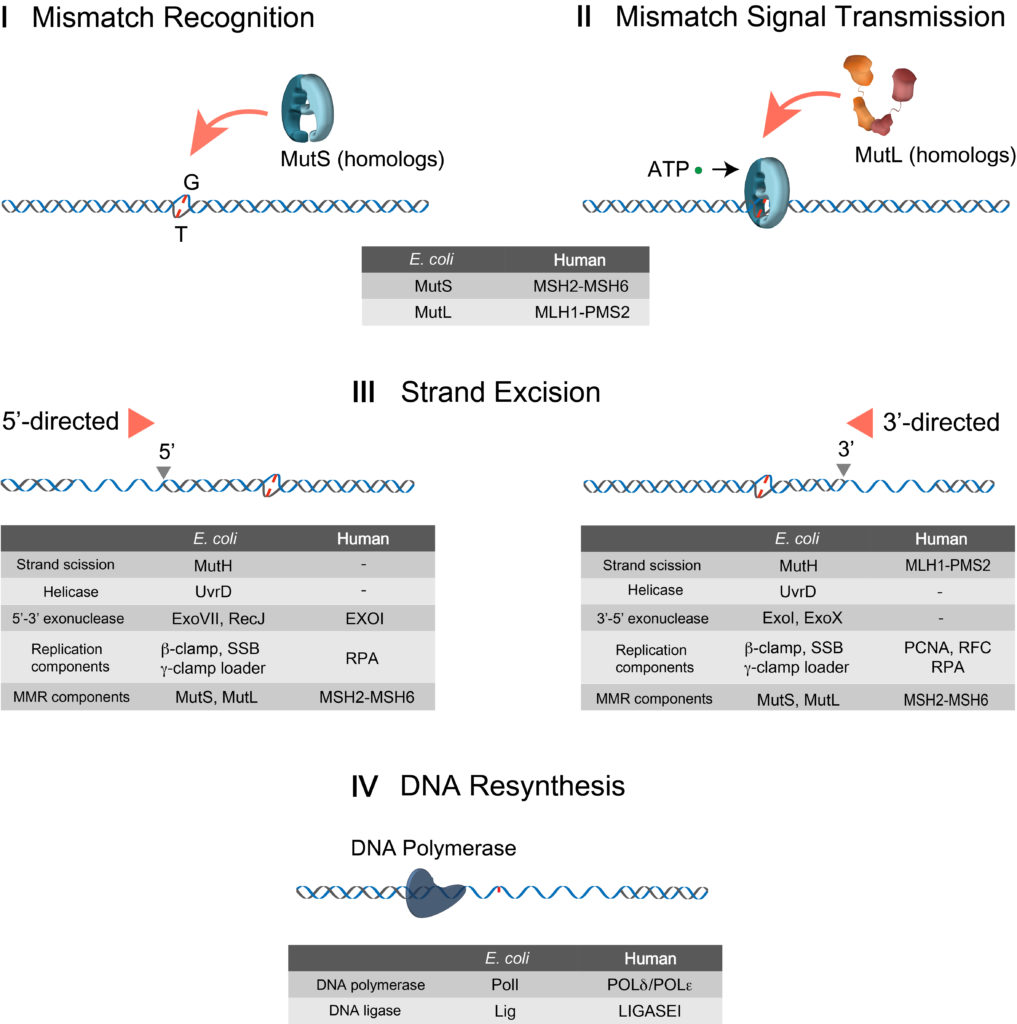
DNA MMR processes: I Mismatch Recognition: MSHs search and recognize the mismatch. ➔ II Mismatch Signal Transmission: Mismatch finding is transmitted to a downstream site by MSH and MLH/PMS, for which ATP is required. ➔ III Strand Excision: Strand excision is initiated at a 5′-end (5′-directed strand excision) or a 3′-end (3′-directed strand excision) to release the mismatch. ➔ IV DNA Resynthesis: After the mismatch is eliminated, DNA polymerase fills the gap and Ligase joins two ends of ssDNAs. Components participating in each MMR process are listed on the table.
Publication: Daehyung Kim et al. Journal of Molecular Biology (2018)
The concept of mismatch repair (MMR) was formulated independently in 1964 to explain the removal of brominated nucleotides from DNA as well as gene conversion during genetic recombination. In the intervening 40 years, the field has developed incrementally, punctuated by a number of transformative genetic and biochemical studies. Two core MMR genes, MutS and MutL, have been conserved throughout life on earth. Defects in human MutS homologues (MSH) and MutL homologues (MLH/PMS) cause the common cancer predisposition Lynch syndrom or hereditary nonpolyposis colorectal cancer (LS/HNPCC). Work on the mechanism of MMR has been significantly aided by completely defined biochemical systems in vitro as well as several crystal snapshots that depict critical intermediates. It has been mired by unseemly biochemical conditions and misinterpretation. The contemporary use of real-time single molecule imaging has the potential to finally and fully resolve the mechanics of MMR.
Translesion synthesis (TLS)
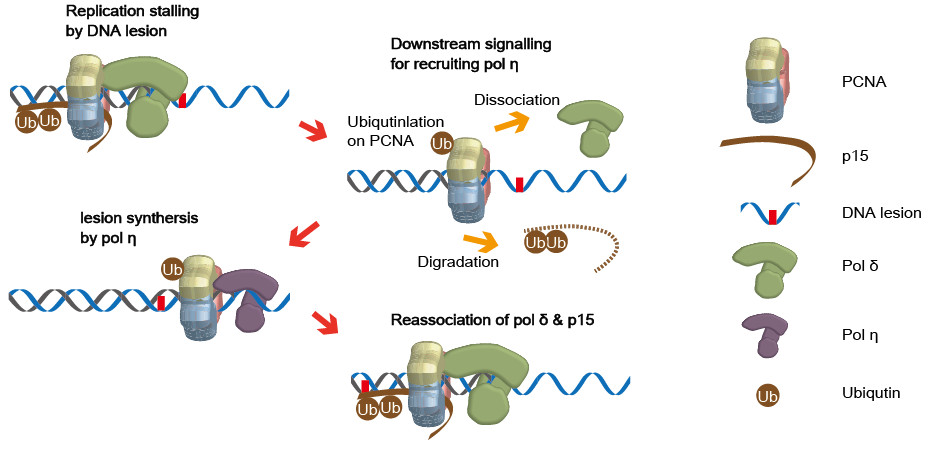
Most of DNA molecules in cells in S phase are replicated by replicative DNA polymerases. Replicative polymerases are fast, progressive, and accurate. However, they cannot synthesize damaged-DNA bases caused by oxidation, chemicals, or Ultraviolet light (UV). The stalling of replicative polymerases on damaged-DNA bases can result in cell death. To avoid this result, cells have DNA translesion synthesis (TLS) process with translesion DNA polymerase for fast resuming replication instead of repairing the damaged DNA bases. When the replisome encounters damaged DNA bases, TLS process switches polymerases from replicative to translesion one. Basically, TLS process is a switching between replication and translesion polymerases. This process is controlled by ubiquitination, degradation, association/dissociation, and diffusion of PCNA and p15. We are visualizing the whole processes using single-molecule methods.
